How efficient is your CO2 storage reservoir?
A common reference point for how well a CO2 reservoir will permanently store that CO2 over geologic time is commonly referred to as its 'storage efficiency'. But what exactly is that? And why is one reservoir type inherently more likely to have a higher storage efficiency than another? Let's dig into both questions, shall we?
1) CO2 Storage Efficiency Rule of Thumb: The 2009 IEA Chart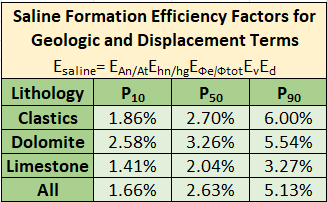
A common starting place for most groups looking to quantify the volumes of CO2 that may be stored in the subsurface is the 2009 IEA Report on this very topic. A link to this report can be found here. This methodology has been also used by the US Department of Energy, and updated methodologies have been applied by various authors. In the original study, the authors detail a method to estimate storage efficiency; the amount of porosity in the target formation that will hold CO2 volumes permanently. The equation to calculate the storage efficiency, as seen in the figure above, holds that saline aquifer storage efficiency will be a function of five variables. Those variables, and what they account for, are:
- EAn/At - the fraction of the aquifer that is suitable for CO2 storage
- EHn/Hg - the fraction of the geological formation that meets requirements of porosity and permeability for CO2 injection and storage (commonly referred to as the "net-to-gross ratio")
- EΦe/Φtot - the ratio of effective porosity to total porosity. Effective porosity is the interconnected porosity that will allow the CO2 and associated pressure front to transmit through it.
- Ev - the vertical displacement efficiency metric that describes the fraction of the vertical aquifer cross section that will be contacted by injected CO2. This measure is a function of aquifer layering and dip and CO2 buoyancy
- Ed - the microscopic displacement factor that represents the fraction of water-saturated pore volume that can be replaced by CO2.
We want to talk about why we see the different values for clastics (e.g. sandstones), dolomites and limestone, respectively. As you will see, not all rocks are created equal, and each rock type has distinct advantages and disadvantages.
2) Clastics
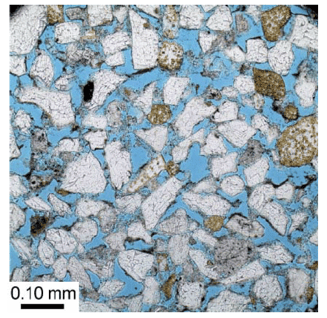
Clastic deposits are made of broken up pieces of rock that are typically sourced from continental regions. As such, these rocks have a lot of silica in them, with lesser amounts of feldspars. The high silica content is important, as it is a relatively durable rock that is less prone to changes after being deposited (a process called "diagenesis"). This means that once a clastic rock is deposited, it usually maintains the reservoir properties that were given to it during deposition, and often times that can be very high reservoir quality. Sands that are well-sorted and well-rounded can have high porosity and permeability, even after burial. Depending on what environment those sands were deposited in, the rocks can be very laterally continuous, and, to a lesser degree, vertically continuous as well. The photo below from Sakurai and others (2009) shows a photo taken from a microscope of a thin slice of the Wilcox sandstone that has been filled with blue epoxy to show the available porosity. As you can see, there is a lot of porosity and it is well connected! This will lead to higher storage efficiencies, and helps to explain the generally higher Esaline values.
3) Dolomites
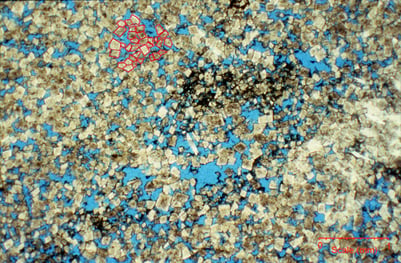
Dolomite deposits form through a process known as dolomitization, which involves the alteration of limestone or lime mudstone into dolomite. Dolomite is a sedimentary carbonate rock composed predominantly of the mineral dolomite (calcium magnesium carbonate, CaMg(CO3)2). Dolomite deposits are fairly common in the rock record, and are typically found in marine and evaporitic environments. They can occur in various geological settings, including ancient reefs, lagoons, tidal flats, and sabkhas (coastal salt flats). These environments provide the necessary conditions for dolomitization to take place, such as the presence of magnesium-rich fluids or hypersaline conditions that favor dolomite precipitation.
Dolomites have higher storage efficiency ranges for several reasons. First, these rocks undergo early cementation that helps to prevent compaction and helps the rock to retain porosity and permeability, as seen in the microscope image below from wonderful work done by Jerry Lucia of the Texas BEG. Secondly, dolomite is chemically stable, and less prone to later diagenesis that could degrade reservoir properties. Lastly, some dolomite are created in depositional environments that are laterally continous, thus increasing the chance that injected CO2 and the associated pressure front will be able to move laterally through the rock formation.
4) Limestones
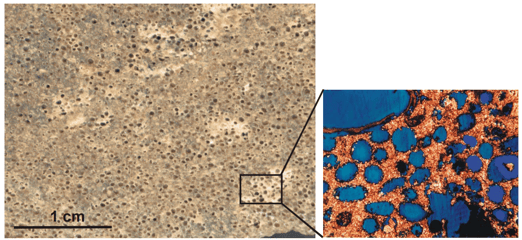
Limestone is a sedimentary rock primarily composed of calcium carbonate (CaCO3) derived from the accumulation and compaction of organic remains (such as shells, coral, algae, and microorganisms) or the precipitation of calcium carbonate from water. It is one of the most abundant rock types on Earth and has various uses in construction, agriculture, and industry. Limestone formation typically occurs in marine or freshwater environments, although some limestone can also be formed in caves through chemical processes.
Unlike the other rock types, limestones are much less chemically stable, and can experience diagenetic alteration almost immediately after deposition. As can be seen in the image below from research performed by the University of Kansas Center for Research, there can be a complete porosity inversion with limestones...this mean that the open space between grains becomes filled with cement as the limestone grains are dissolved. There may be abundant porosity in this example, but it is not well connected and will be more difficult to inject CO2 into at high rates compared with more connected pore systems. Also, limestones can be more susceptible to compaction than the other rocks, particularly during deep burial, and but is also quite brittle, leading to fractures. Fractures provide ample permeability in the formation, but the CO2 may bypass the other forms of porosity in the limestone as it follows the path of least resistance, resulting in lower storage efficiency.
So...how do you tell how efficient YOUR reservoir will be? You need a trusted geoscience advisor that has worked with these kinds of reservoirs before to provide the proper guidance. At The GeoIntegra Group, we have years of experience working with all of these reservoir types. Reach out to us today to see how we can help you achieve your CO2 storage goals!

 By
By