Why it's super-critical that your underground CO2 is supercritical
You may have heard that CO2, when stored underground, needs to be maintained at supercritical conditions. But what the heck is 'supercritical'. Moreover, why is important...or super-critical, if you will? Let's dig into that very question.
The basics: What is Supercritical CO2?
As can be seen in the chart below, CO2 can exist as several different fluid phases as a function of pressure or temperature. At very low temperatures and pressures, CO2 can either have a gaseous or solid form (think dry ice). However, CO2 above the triple point a third phase is introduced: liquid CO2. As pressures and temperatures rise, a critical point is reached at about 304 deg K, 31 °C, or 88 °F and critical pressure 7.4 MPa, 73atm, 1,070 psi, or 74 bar, depending on which flavor of pressure measurement you prefer. Beyond this temperature and pressure domain an entirely new phase shows up: supercritical CO2.
What is so special about CO2? Read on to learn why...
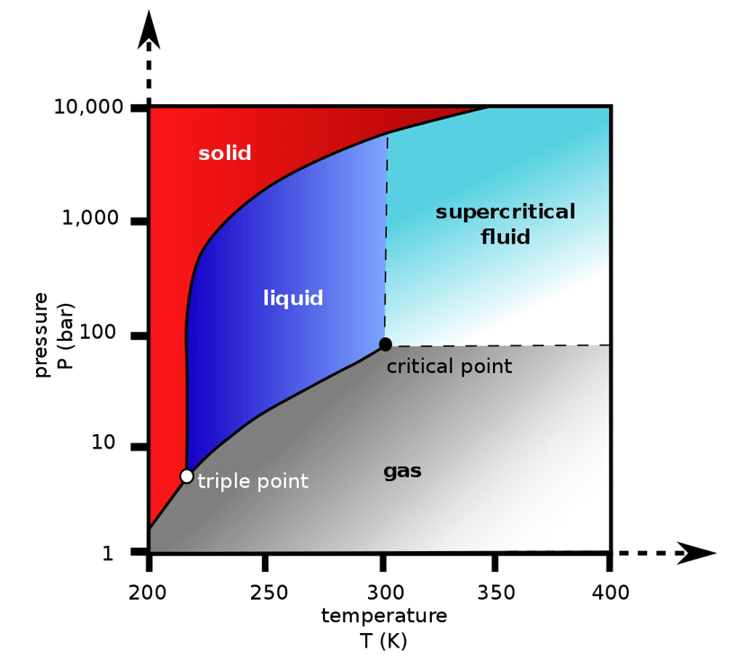
Image Source: Wikipedia
What is so special about supercritical CO2? Density!
As mentioned, the fluid phase of CO2 will be dependent upon temperature and pressure. At normal atmospheric conditions, CO2 will be in a gaseous phase. However, as CO2 is captured at the surface and injected into underground reservoirs (a process called CCS or CCUS...click here to learn more about the difference), that CO2 will change as it experiences at higher temperatures and pressures. When the CO2 reaches the supercritical temperature and pressure, it will become a much denser phase than it was at the surface. This is important because CO2 storage projects will be more successful at storing large volumes of CO2 if they are stored in a dense phase. After all, light and fluffy CO2 works for us at the surface, but dense and compact is the way to go when your deep below. How deep do you have to get for CO2 to become supercritical? It depends on the temperature and pressure profile where you inject that CO2, but it is typically about 2600 feet (790m) below the surface of the earth.
So is that all that makes supercritical CO2 special? Nope...there's more.
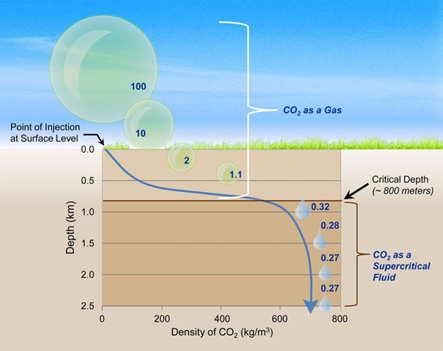
Image Source: NETL
What is so special about supercritical CO2? Viscosity!
Gaseous forms typically have much lower viscosities than their gaseous counterparts. The interesting thing about supercritical CO2 is that it has a relatively low viscosity as both a gas and a supercritical liquid. As can be seen in the figure below, CO2 density and viscosity (in red) both increase as a function of depth. CO2 viscosities also increase with depth, but are still at a lower viscosity than water (in blue), as seen in the plot on the far right of the figure. Why does this matter? If one seeks to inject C02 into a water bearing reservoir (which describes most CCS targets), it is very useful that the CO2 is less viscous than the water inside the target reservoir. This will allow for easier migration of fluids away from the injection site and into the confining reservoir interval.
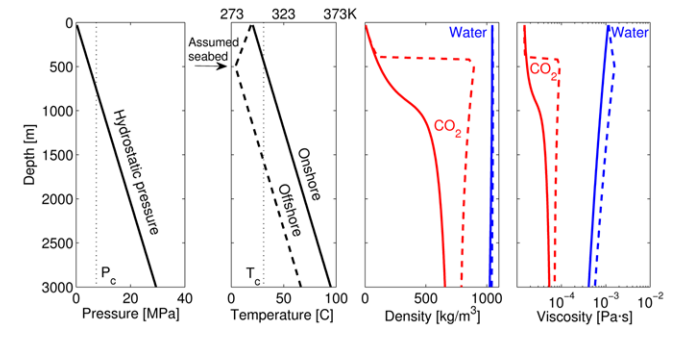
Image Source: Espinoza et al., 2011. DOI 10.1007/s12205-011-0011-9
That's it for now. Hope you found this useful!

 By
By